Page content
Impact
The development of globally relevant healthcare technologies for the benefit of patients and the economy is a core theme in the Ulster Engineering research portfolio. Our research is intricately linked with international partners in both the commercial and clinical spheres.
It has impacted local government policy through our leadership in forums that include MATRIX and Innovate UK/Health KTN. We have also witnessed global impact through our representation in international scientific forums such as Computing in Cardiology, the International Society for Computerised Electrocardiology, and our ECG signal processing algorithms have been adopted by the United States Food and Drug Administration (FDA).
Collectively, this activity has resulted in the growth of the GBP9,300,000 industry-focused Connected Health Innovation Centre (CHIC) established at Ulster, the EUR8,400,000 Eastern Corridor Medical Engineering Centre (ECME), the GBP7,300,000 industry-focused Biodevices Rapid Prototyping Laboratory and the showcasing of our research on the global stage through our team’s 3rd place success in the XPRIZE Tricoder competition.
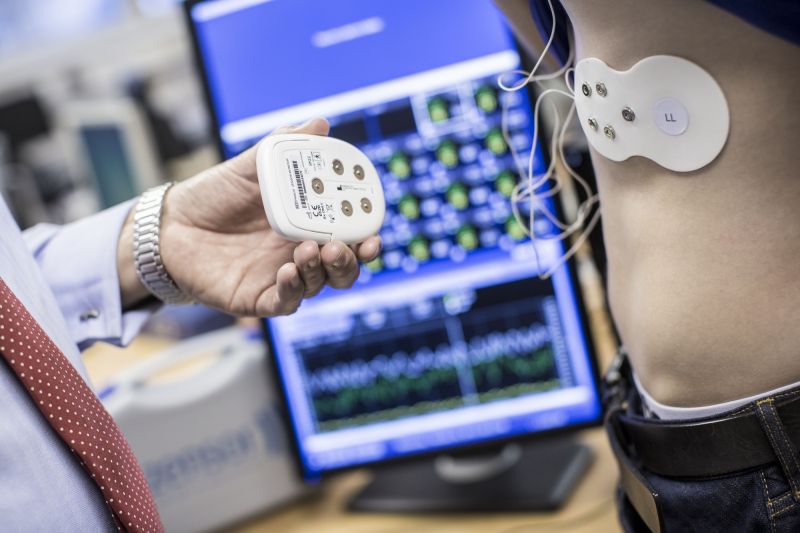
Our research has recently resulted in the sale of the successful Ulster spin-out company Intelesens to Ultralinq (2017). The commercialisation of this cardiovascular monitoring technology has had a beneficial impact on health costs and patients’ lives on a global scale over the past seven years.

Ulster has an extensive record in the development of healthcare sensor systems and is unique in its ability to deliver on the entire technology stack required in the development of end-toend, ‘sensor-to-diagnosis', healthcare technology devices. These activities range from the design, development, and fabrication of physical sensing electrodes to the development of software algorithms for highly accurate automated diagnosis and decision support. A key specific theme in Nanotechnology and Integrated Bioengineering Centre’s (NIBEC) record of accomplishment over the past 25 years has been major advancements in the acquisition and analysis of cardiac bio-signals.

- Press article relating to Intelesens device FDA approval: https://www.mobihealthnews.com/47424/fda-clears-intelesenss-zensor-a-wearable-vitalsmonitor
- Operations Director – Intelesens/Ultralinq, to provide account of impact of Ulster research in development of monitoring solution and subsequent impact on company growth/revenue streams.
- Summer 2014 issue of the “The British Private Equity & Venture Capital Association (BVCA)” Journal listing Intelesens as winner of “Best for Innovation” award https://www.bvca.co.uk/Portals/0/library/documents/Journals/BVCA%20Journal%20Summer%20 2014.pdf
- Press article in BBC announcing UltraLinq’s acquisition of Intelesens in 2017: https://www.bbc.com/news/uk-northern-ireland-40817009
- Press article relating to comments by the COO of UltraLinq, on recognising role of Ulster University in decision to acquire Intelesens Ltd. https://www.belfasttelegraph.co.uk/business/northern-ireland/medical-tech-firm-intelesens-setssights-on-growth-after-us-buyout-35998405.html
- Press article acknowledging Ulster team's 3rd place in Tricorder XPRIZE competition. https://www.siliconrepublic.com/machines/ulster-university-tricorder-prize
- CEO and Founder of PulseAI (https://www.pulse-ai.co.uk/), Recent University related startup, to provide details of Ulster support and collaboration in start-up.
- Official letter from the US FDA, outlining the collaboration between Ulster and the FDA.
- Navarro-Paredes, C., Kurth, M.J., Lamont, J.V., Menown, I.B., Ruddock, M.W., Fitzgerald, S.P. and McLaughlin, J., 2018. Diagnostic Performance of a Combination Biomarker Algorithm for Rule-Out of Acute Myocardial Infarction at Time of Presentation to the Emergency Department, Using Heart-Type Fatty Acid-Binding Protein and High-Sensitivity Troponin T tests. Journal of Clinical and Experimental Cardiology, 9(8), pp.1-9.
- CEO/Founder of CIGA Heathcare Ltd and UK-RTC consortium member, to provide account of Ulster’s impact in the development of Lateral Flow technology for Ciga healthcare and Ulster’s input to the UK-RTC
Contributors
- Professor James McLaughlin
- Professor John Anderson
- Professor Dewar Finlay
- Professor Omar Escalona
- Dr Daniel Guldenring
- Dr Patrick Lemoine